Washington State University researchers have found a way to more efficiently create hydrogen from water – an important key in making renewable energy production and storage viable.
The researchers, led by professors Yuehe Lin and Scott Beckman in the School of Mechanical and Materials Engineering, have developed a catalyst from low cost materials. It performs as well as or better than catalysts made from precious metals that are used for the process.
Washington State University researchers have found a way to more efficiently create hydrogen from water – an important key in making renewable energy production and storage viable.
The researchers, led by professors Yuehe Lin and Scott Beckman in the School of Mechanical and Materials Engineering, have developed a catalyst from low cost materials. It performs as well as or better than catalysts made from precious metals that are used for the process.
The work is published in the journal Advanced Energy Materials (http://onlinelibrary.wiley.com/doi/10.1002/aenm.201601555/full).
Storing clean energy
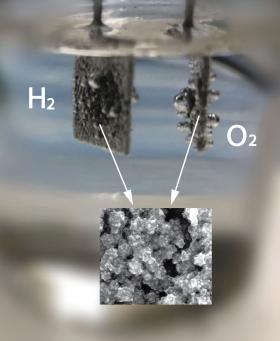
Gas bubbles form as researchers use a unique catalyst to convert water to hydrogen and oxygen. The inset image shows the catalytic materials at the nanoscale.
Energy conversion is a key to the clean energy economy. Because solar and wind sources produce power only intermittently, there is a critical need for ways to store and save the electricity they create.
One of the most promising ideas for storing renewable energy is to use the excess electricity generated from renewables to split water into oxygen and hydrogen; the hydrogen can then be fed into fuel-cell vehicles.
Continue reading at the Washington State University - Pullman
Image: Gas bubbles form as researchers use a unique catalyst to convert water to hydrogen and oxygen. The inset image shows the catalytic materials at the nanoscale.
Image via Washington State University - Pullman