Rice University scientists have created an efficient, simple-to-manufacture oxygen-evolution catalyst that pairs well with semiconductors for solar water splitting, the conversion of solar energy to chemical energy in the form of hydrogen and oxygen.
Rice University scientists have created an efficient, simple-to-manufacture oxygen-evolution catalyst that pairs well with semiconductors for solar water splitting, the conversion of solar energy to chemical energy in the form of hydrogen and oxygen.
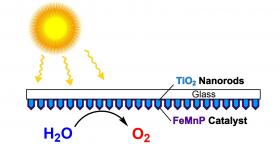
The lab of Kenton Whitmire, a Rice professor of chemistry, teamed up with researchers at the University of Houston and discovered that growing a layer of an active catalyst directly on the surface of a light-absorbing nanorod array produced an artificial photosynthesis material that could split water at the full theoretical potential of the light-absorbing semiconductor with sunlight.
An oxygen-evolution catalyst splits water into hydrogen and oxygen. Finding a clean renewable source of hydrogen fuel is the focus of extensive research, but the technology has not yet been commercialized. See more at: http://news.rice.edu/2017/03/23/artificial-photosynthesis-steps-into-the-light/#sthash.krKJ4mV1.dpuf
Image: Scientists at Rice University and the University of Houston created a catalyst from three elements – iron, manganese and phosphorus – and then coated it evenly onto an array of titanium dioxide nanorods to create a highly efficient photoanode for artificial photosynthesis. Click on the image for a larger version. (Courtesy of the Whitmire Research Group)