Berkeley Lab scientists discover critical role of nanoparticle transformation
Scientists at the Department of Energy’s Lawrence Berkeley National Laboratory (Berkeley Lab) have developed a new electrocatalyst that can directly convert carbon dioxide into multicarbon fuels and alcohols using record-low inputs of energy. The work is the latest in a round of studies coming out of Berkeley Lab tackling the challenge of creating a clean chemical manufacturing system that can put carbon dioxide to good use.
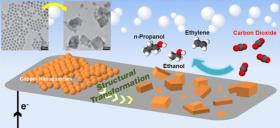
In the new study, published this week in the Proceedings of the National Academy of Sciences, a team led by Berkeley Lab scientist Peidong Yang discovered that an electrocatalyst made up of copper nanoparticles provided the conditions necessary to break down carbon dioxide to form ethylene, ethanol, and propanol.
All those products contain two to three carbon atoms, and all are considered high-value products in modern life. Ethylene is the basic ingredient used to make plastic films and bottles as well as polyvinyl chloride (PVC) pipes. Ethanol, commonly made from biomass, has already established its place as a biofuel additive for gasoline. While propanol is a very effective fuel, it is currently too costly to manufacture to be used for that purpose.
To gauge the energy efficiency of the catalyst, scientists consider the thermodynamic potential of products – the amount of energy that can be gained in an electrochemical reaction – and the amount of extra voltage needed above that thermodynamic potential to drive the reaction at sufficient reaction rates. That extra voltage is called the overpotential; the lower the overpotential, the more efficient the catalyst.
Continue reading at Lawrence Berkeley National Laboratory
Image via Dohyng Kim, Lawrence Berkeley National Laboratory