The fight against climate change is a call-to-arms for industry. We currently rely on fossil fuels, a major source of the greenhouse gas CO2, not only for energy but also to create chemicals for manufacturing. To ween our economies off this dependency, we must find a new source of “green” raw materials so that factories and laboratories can run without producing and emitting CO2.
The fight against climate change is a call-to-arms for industry. We currently rely on fossil fuels, a major source of the greenhouse gas CO2, not only for energy but also to create chemicals for manufacturing. To ween our economies off this dependency, we must find a new source of “green” raw materials so that factories and laboratories can run without producing and emitting CO2.
Now, a research team at Osaka University has discovered how to create valuable chemicals from clean sources. They used biomass, essentially waste from plant materials. Biomass is rich in organic molecules – long chains of carbon atoms attached to oxygen. Existing methods can break the carbon–oxygen bonds in these molecules to create, for example, raw materials for plastics. However, breaking the carbon–carbon bonds, in order to shorten the molecular chains, is harder; extreme temperatures are needed, and often yield unwanted products.
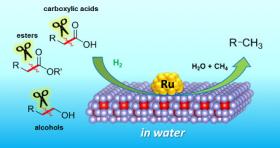
The method developed at Osaka is based on a new catalyst. Catalysts allow reactions to occur, without being consumed themselves. They are often based on metals, and the new example is no exception – it consists of atomically small particles of ruthenium, a metal related to iron, sitting on a material called cerium oxide.
Continue reading at Osaka University
Image: Selective C-C bond cleavages of biogenic chemicals catalyzed by the CeO2-supported ruthenium nanoparticle catalyst (Image Credit: Osaka University)