The market for antibacterial lotions, soaps, and wipes has exploded, and antimicrobial compounds are now common in other consumer items like shampoos, deodorants, shoes and apparel, and food-preparation and storage items, despite widespread evidence that these compounds often don’t work as advertised. But now even our buildings are getting in on the trend. The use of chemicals in building products, especially to kill mold, is centuries-old, but antimicrobial chemicals are proliferating in heretofore rarely seen places: furniture, flooring, wallcoverings, textiles, countertops, sunshades, doorknobs and push-plates, ductwork, and caulking. This article examines applications of antimicrobials in buildings, asking whether they are warranted, and looking at how antimicrobials work. It also explores health and safety concerns and suggests ways to make buildings more hygienic, with or without antimicrobials.
Fear of infection is hardly a new phenomenon, but it seems to have risen to a fever pitch in recent years. Modern medicine appeared to have all but conquered infectious disease decades ago—but in the last three decades our society’s confidence in that victory has unraveled. Diseases like AIDS, anthrax, “mad cow disease,” severe acute respiratory syndrome, antibiotic-resistant tuberculosis, and bird flu have perplexed and challenged the medical establishment, and popular culture and the press have seized on reawakened fears of uncontrollable diseases, reporting on health emergencies around the globe with ever greater fervency.
The market for antibacterial lotions, soaps, and wipes has exploded, and antimicrobial compounds are now common in other consumer items like shampoos, deodorants, shoes and apparel, and food-preparation and storage items, despite widespread evidence that these compounds often don’t work as advertised. But now even our buildings are getting in on the trend. The use of chemicals in building products, especially to kill mold, is centuries-old, but antimicrobial chemicals are proliferating in heretofore rarely seen places: furniture, flooring, wallcoverings, textiles, countertops, sunshades, doorknobs and push-plates, ductwork, and caulking.
To say that cultural fears are driving these trends would be simplistic, however. In buildings, mold growth has long been known to compromise the structural integrity of buildings, and research over the last decade has increased concerns about mold’s effects on indoor air quality. Providing sanitary conditions in homes and commercial buildings alike has become a greater focus in preventing the spread of disease-causing pathogens. Hospitalization is a leading cause of death in the U.S.; among other hazards, infections acquired during hospital treatment kill more than 80,000 people annually (nearly twice the number of people killed in automobile accidents), leading to strong interest in antimicrobials from healthcare facilities.
Some of these infections are caused by “superbugs,” pathogens that have developed resistance to multiple antibiotics used in medicine. (Antibiotics differ from antimicrobials in being specifically medicinal in nature and often being based on compounds derived from fungi and bacteria that are capable of killing fellow microbes.) Similar concerns associated with widespread use of antimicrobials suggest that bacteria could evolve resistance to them, becoming more deadly. The inherent toxicity of antimicrobial compounds—many of which are pesticides—also raises concerns that treatments to prevent disease could cause other problems.
This article examines applications of antimicrobials in buildings, asking whether they are warranted, and looking at how antimicrobials work. It also explores health and safety concerns and suggests ways to make buildings more hygienic, with or without antimicrobials.
!ADVERTISEMENT!
Microbes—and Antimicrobials
The Freedonia Group, a market research firm, projects that U.S. demand for disinfectant and antimicrobial chemicals will increase 5% annually to $930 million in 2009, up from $730 million in 2004. Those dollar figures represent growth in sales from 238 million pounds (108 million kg) in 2004 to 273 million pounds (124 million kg) in 2009. This growth is spread across several categories, with paints and coatings accounting both for the greatest volume compared with other categories, such as plastics and healthcare, and some of the strongest growth. Another product category, copper-based biocides, most commonly used as wood preservatives, accounted for another $245 million in sales in 2005, according to Freedonia, and shows similarly strong growth. (For more on protecting wood from insects and mold, which is not discussed in this article, see EBN Vol. 15, No. 8).
Bacteria
The growth and spread of these chemicals pales, of course, in comparison with microbes, which are much more widespread and can proliferate much faster in a given environment. Bacteria typically require moisture, a food source, and adequate warmth. One environment providing those factors in abundance is the human body: scientists have estimated that 500 to 1,000 species of bacteria live on and inside us, with ten times as many bacterial cells as human cells. Most of those bacteria live in the large intestine, where they help us digest food.
Humans require the same things in their environment as bacteria—moisture, food, and warmth—so we tend to create ideal bacterial habitats all around us. Furthermore, humans frequently move around in bacteria’s environments touching ourselves, each other, and many other surfaces along the way—creating many ways for bacteria, viruses, and other microbes to spread.
Mold
Fungi are distinct from bacteria: they inhabit separate taxonomic kingdoms, and some of the best known fungi and bacteria are adversaries: the Penicillium mold, which kills and inhibits the growth of some bacteria, was the first modern antibiotic. However, fungi can interact with humans and the environment in similar ways to bacteria, leading to some similar problems.
American Clay Earth Plaster, shown with a troweled finish in this bathroom, has inherent mold-inhibiting properties, according to the company, because it naturally releases moisture.
Of greatest concern in terms of the built environment are molds, which grow on and decompose organic material, including wood, paper, and cloth. Dirt, which usually contains a high proportion of organic material, also offers a home to mold, allowing it to grow on surfaces that would otherwise not support it, such as concrete, glass, and metal. Mildew is a form of mold, as is the foggy substance that frequently grows on the outside of windows.
Whether mold exposure causes widespread health problems is a largely unresolved scientific question (see more in EBN Vol. 10, No. 6). Summing up the evidence in a 2002 paper that remains relevant today, building scientists Nathan Yost, M.D., Joseph Lstiburek, Ph.D, P.Eng., and Terry Brennan wrote, “Most people are not affected by exposure to mold, unless they are exposed to a lot of mold. Unfortunately, we are not quite sure what ”˜a lot of mold’ means.” Mold’s decomposition and despoliation of building materials poses practical and aesthetic concerns. Prevention of mold growth in interiors is an important goal—can antimicrobials help?
Antimicrobials
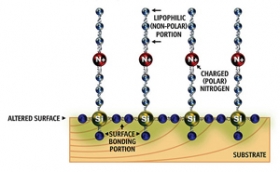
In order to be effective against microbes that are as ubiquitous and small as mold and bacteria, antimicrobial substances need to be similarly ubiquitous in distribution and have properties making them effective at the cellular level.
The Microban brand of antimicrobial products offers an example of how antimicrobials are incorporated into products and how they work, as well as how they cause health and environmental concerns. Microban International, based in North Carolina, develops antibacterial formulations for product manufacturers. Wayne Swofford, head of research and development for Microban, explained that Microban antimicrobials typically will be “durably incorporated into whatever product is going to be used or sold. The specific type of antimicrobial is going to vary based on the end use and the matrix you’re putting it into,” he said. Incorporating Microban into a product allows the microbial to provide “continuous action, versus periodic treatment of that surface,” said Swofford.
In building products, Swofford said, antifungal protection is usually a top priority, leading Microban to use products including pyrethiones, isothiazolinones, and azoles. Microban frequently incorporates triclosan and has become best known for that chemical. Triclosan, found in toothpastes, mouthwashes, and soaps, as well as numerous other hygiene products, apparel, and interior finishes, works by inhibiting various bacterial functions. At the lower concentrations typical for building products, triclosan is a biostat, or an inhibitor of growth in bacterial populations; it works by interfering with the synthesis of fatty acids, which are needed for building cell membranes.
Environmental Risks
Although a chemical like Microban offers clear benefits—continuous, targeted treatment for bacteria in infection-prone situations—numerous medical and environmental groups have raised questions about risks. These groups warn about health and environmental risks from antimicrobials, including antimicrobial resistance, and exposure, both direct and mediated through the environment.
Antimicrobial resistance
Bacterial resistance to antibiotics is a well-established problem in public health. Staphylococcus aureus, for example, is the main bacterium responsible for staph infections and a range of diseases, many of them fatal. Staphylococcus developed resistance to penicillin in 1947, four years after penicillin was first mass-produced. Staph bacteria resistant to multiple antibiotics have since developed and become common. This history contributes to widespread fear, articulated by the American Medical Association and others, that bacteria targeted by antimicrobials could adapt and develop resistance to those chemicals, as has happened in medicine with antibiotics.
Resistance to silver
Silver is a powerful antibacterial, and it is commonly used in medical settings as well as in building materials. Silver kills bacteria by interfering with their cell membranes, causing internal fluids to be released and allowing entry of the silver, which can then kill the bacterium in several ways, including binding with proteins and killing enzymes. Milliken uses a silver-based antimicrobial on all of its carpeting, and silver is an option in ductwork, washing machines, and mobile phones, among other applications.
Silver also provides a case study in antimicrobial resistance. According to Simon Silver, Ph.D., professor of microbiology at the University of Illinois-Chicago, bacteria resistant to silver appear regularly, as many as a few times a year in different places, and “highly resistant” bacteria have also appeared at times, including in the 1970s in a Boston burn ward. In that case, following three deaths from resistant Salmonella bacteria, the ward was closed and intensely cleaned, eliminating the problem. The saving grace in these cases is that, for whatever reason, neither the silver-resistant bacteria nor the genes responsible for their resistance seem to have spread.
Although not discounting the danger entirely, Silver argues that use of silver in buildings isn’t likely to cause antimicrobial resistance. He says that the metal has been commonly used for so long in applications where it contacts bacteria, including dental fillings, jewelry, food utensils, and photography, that its new uses in buildings are relatively minor.
This Bostik coating, incorporating AgION antimicrobial silver, is applied to freshly poured concrete prior to installation of floorcovering to prevent moisture transmission.
Nonetheless, especially since bacterial resistance to silver has been shown to occur, the risk should be properly disclosed, an action at least one manufacturer appears not to take. Ginger Merritt, vice president of worldwide marketing and sales for Agion Technologies, makers of silver-based antimicrobial AgION, told EBN that “silver works in three ways. ”¦ Over time the microorganism can learn its way around one mechanism. With three it’s unlikely if not impossible.”
The past emergence of silver-resistant bacteria shows that Agion may be underrepresenting the risks. Moreover, according to Silver, “The mechanism of resistance has nothing to do with the mechanism of action.” That is, bacteria don’t have to “learn their way around” multiple mechanisms of attack to form a counterattack. Bacteria resist silver by binding it to the outside of their cells, or pumping it out as fast it enters, said Silver.
Another danger that is frequently underplayed by manufacturers touting the safety of their own antimicrobials is cross-resistance. “Microbes package their resistances on little pieces of DNA called plasmids,” said Silver. “With that package a bug will gain resistance to a whole bunch of different things.” Bacteria can also exchange those plasmids with each other, potentially spreading resistance. In addition to the gene-packaging issue contributing to cross-resistance is the possibility that a mechanism for resistance could apply to multiple chemicals, said Bernie Weisblum, M.D., professor of pharmacology at the University of Wisconsin”“Madison. “One common way of expressing resistance is for the bacterium to pump [the antimicrobial] out, and some of the pumps may have a general activity,” he said. Indeed, the silver-resistant bacteria that appeared in Boston were also resistant to ten other antibiotics, Silver said.
Even with this history of silver resistance in bacteria, evidence doesn’t link instances of resistance to antimicrobial use in buildings, and other common antimicrobial chemicals remain unlinked to resistance. Stuart Levy, M.D., a professor of microbiology at Tufts University who has studied antimicrobial resistance, said that concerns remained, particularly with chemicals that leave residues. “That leaves out alcohols, peroxides, and bleaches but leaves in those compounds that contain triclosan, triclocarbon, and QACs [quaternary ammonium compounds],” he said. “The volatile compounds do their killing immediately and leave no residual substance to allow other bacteria to become trained to be resistant,” Levy explained.
Exposure hazards
The factors that make antimicrobials effective—including their toxicity and their need to be fairly durable—can make them hazardous to people and the environment. Here we briefly examine several common compounds—silver, triclosan, and zinc pyrithione—and their potential hazards.
Silver: Proponents of the use of silver, like Agion’s Merritt, call it a “naturally occurring chemical.” Others, meanwhile, call it a “toxic heavy metal.” They’re both technically correct, requiring us to carefully sort out hype from reality. Silver has been used in medicine for decades, and, while doctors should take care not to overuse it, most scientists would probably agree with microbiologist Silver when he told EBN that “silver’s toxicity for humans is very little and only in a few situations.” According to Silver, a sterile wound will heal faster using bandages not impregnated with silver. However, those bandages protect wounds from the very real risk of infection—ultimately leading to faster healing, especially for burns and other severe wounds. An additional concern is that antimicrobial silver is ultimately released to the environment, where it could disrupt aquatic life, including bacterial populations. However, there has so far been little evidence to support this fear.
Triclosan: Triclosan is relatively nontoxic to humans and other mammals, in part because humans synthesize fatty acids with a process different from that used by targeted bacteria. However, triclosan appears to be very persistent in the environment: a 2002 study by the U.S. Geological Survey found triclosan in 58% of the natural waters it tested that year. In addition to its possible role as an endocrine disruptor, as one study has shown in frogs, triclosan can convert to a potentially toxic dioxin when exposed to sunlight, according to a 2003 study (see EBN Vol. 12. No. 7). Triclosan also appears to be bioaccumulative, so additional problems could emerge as it becomes more widespread and studied in more contexts.
Zinc pyrithione: Zinc pyrithione is primarily a fungicide with some antibacterial properties. It is common in anti-dandruff shampoos and in building products as a mildewcide and algaecide in paints and wet-applied adhesives (where it has become more common as solvent-based adhesives have been replaced by waterborne formulations, in part because of air-quality concerns from solvents’ offgassing). In higher doses zinc pyrithione is acutely toxic to humans, but at the doses present in building materials—doses much lower than those in shampoos, according to industry sources—it does not appear to be toxic.
Effectiveness Unproven
Duraban offers an antimicrobial coating for a variety of surfaces using quaternary ammonium compound. The chemical works by using a positively charged molecule with a carbon-chain “sword” to attract and impale bacteria, which have negatively charged cell walls. Although the compounds are relatively nontoxic, some experts worry that bacteria could develop resistance to them through constant exposure.
While many antimicrobials appear to be relatively safe to occupants, whether they are effective is another question. The immediate efficacy of antimicrobials in killing microbes is not a matter of debate: the U.S. Environmental Protection Agency (EPA) requires companies registering pesticides to show that they work in a given application. However, it’s not as clear that antimicrobials remain as effective in real-world applications as they are in lab tests, or that their use creates overall benefit.
Healthcare authorities skeptical
Antimicrobial products are prevalent in healthcare settings, providing an arena to evaluate their effectiveness. So far, healthcare authorities have found a lack of supporting evidence and have adopted a precautionary approach. One report, “Guidelines for Environmental Infection Control in Health-Care Facilities,” published by the federal Centers for Disease Control and Prevention (CDC) in 2003, offers one of the most exhaustive examinations to date of the evidence for and against the use of antimicrobial compounds in interiors.
Discussing clothing, linens, and mattresses treated with antimicrobials, the CDC report concludes, “No evidence is available to suggest that use of these products will make consumers and patients healthier or prevent disease. No data support the use of these items as part of a sound infection-control strategy.” The report came to this conclusion despite extensive evidence it cited for potential contamination of linens and beds, particularly wet mattresses. But the agency recommends simply following cleaning and sanitizing procedures, and removing damaged mattress covers and mattresses from service.
The CDC report provides another case study, taking a closer look at carpeting (see also EBN Vol. 16, No. 6). “Several studies have documented the presence of diverse microbial populations, primarily bacteria and fungi, in carpeting,” it reports, going on to say that “new carpeting quickly becomes colonized, with bacteria growth plateauing after about four weeks. Vacuuming and cleaning the carpeting can temporarily reduce the numbers of bacteria, but these populations soon rebound and return to pre-cleaning levels.” The report notes that bacterial contamination tends to increase with higher levels of activity and that soiled, damp carpeting provides ideal conditions for bacteria and fungi to proliferate.
Despite all this, “only limited epidemiologic evidence demonstrates that carpets influence health-care-associated infection rates in areas housing immunocompetent patients,” or patients with healthy immune systems, the report concludes. If, as the report concludes, there is no link between infection rates and carpets, then one would expect carpets treated with antimicrobials to have little positive impact. The report makes this point, noting that “treated carpeting has not been shown to prevent the incidence of health-care-associated infections in care areas for immunocompetent patients.”
Regulations from the U.S. Occupational Safety and Health Administration (OSHA) and EPA further suggest that there is not a significant difference between how carpets with and without antimicrobial treatment fare in real-world use. The antimicrobial effect that chemicals provide at the surface of a material as it comes in contact with an infected substance does not extend throughout a substance spilled on the material, especially a viscous, potentially infectious fluid like blood or sputum. An OSHA ruling speaks to this crucial gap in effectiveness. OSHA requires that in occupational settings, following contact with potentially infectious substances, equipment and surfaces be cleaned and disinfected. However, the EPA has not approved a test protocol for how a chemical could be shown to disinfect carpeting, and therefore pesticides registered as disinfectants apply only to hard surfaces, not to carpeting. (EPA distinguishes between antiseptics, sanitizers, disinfectants, and sterilizers, which are increasingly effective in killing microbes in a given application.) OSHA has noted that because EPA-registered pesticides, including all of those used as antimicrobials in carpet, cannot disinfect carpet, it follows that carpet cannot be disinfected following contamination.
Researchers at Kaiser Permanente, the nonprofit managed-care giant, came to a similar conclusion as the CDC authors in a December 2006 memorandum, “Evaluation of Antimicrobial Property Claims in Finishes and Fabrics.” Analyzing how infectious diseases are transmitted, the memo notes that in a “chain” of transmission, one broken link prevents infection. Emphasizing that building environments can frequently be recontaminated, the report argues that handwashing by medical personnel is most effective in breaking the chain of transmission. Citing logic similar to OSHA’s, the Kaiser memo also argues that mechanical cleaning with detergent and water is most effective in controlling contamination of surfaces. The memo further argues that antimicrobial treatments of surfaces are not effective in preventing airborne distribution of disease. The memo concludes, “Review of current scientific literature reveals no evidence that environmental surface finishes or fabrics containing antimicrobials assist in preventing infections.”
What EPA registration means
The apparent disconnect between the skeptical approach of these health and safety authorities and the claims of manufacturers can be better understood through an examination of the EPA pesticide registration process.
In addition to ensuring that an antimicrobial product doesn’t harm human health or the environment, among other requirements, EPA requires data from manufacturers demonstrating that the product is effective against targeted bacteria, fungi, or viruses. Most building products fall under EPA rules that limit manufacturers to claiming protection of the product from pests; expressed or implied public health claims are not allowed. For example, many water-based paints contain antimicrobials, usually to prevent mildew and algae growth on the paint while it is in the can or on a building. The benefit to the consumer is that the paint remains good to use and, after application, does not decay or discolor.
Claims often blur the line, however, in terms of whether antimicrobials simply protect the product or protect public health. Interface, for example, in a technical brief on Intersept, the antimicrobial it uses in all of its carpet tiles, emphasizes the “protection” Intersept offers to its products as a “microbial-inhibiting preservative.” The same brief claims that by providing that protection, “Intersept protects the quality of the indoor environment”—which could be interpreted as a claim regarding public health. Daniel Price, Ph.D., director of microbiology for Interface, told EBN that “Intersept is there to protect the product,” noting that Intersept protects a plasticizer in Interface’s carpet tile backing that could be a fungal food source. Price also extended his argument into public health, however: “We make no health-related claims even though it stands to reason that if I’m not living an a mold-laden environment, I might be better off,” he said.
Interface uses images like this one to advertise the antimicrobial protection afforded by its use of Intersept, a registered pesticide, in its carpet tiles. With the two carpet samples spread with an agar solution and exposed to heat and humdity, the Intersept-treated carpet sample on the right does not grow mold.
Dennis Edwards, chief of the regulatory branch in the EPA’s antimicrobials division, indicated that EPA frowns on this kind of marketing: “The claims have to be limited to the fact that the carpet has been treated to protect the carpet from stains and odors, or to keep it from breaking down,” he said. “The articles don’t need to be protected from diseases—people do,” he added. (When asked why Interface linked Intersept to indoor environmental quality, Price emphasized Intersept’s odor-preventing qualities.)
Erica Stewart, a certified industrial hygienist, a project manager with Kaiser Permanente, and a coauthor of Kaiser’s memo quoted in this article, described what she has seen in antimicrobial product marketing: “What happens in marketing is that manufacturers conflate the antimicrobial label with claims that it will also prevent infection. When we’ve asked for studies, what we’ve actually received from manufacturers were testimonials, no actual well-designed studies.”
Since EPA requires efficacy testing, those buying products with antimicrobials should at least be able to know that the product will be protected. That is likely true in most cases, but buyers should understand the difference between laboratory testing and the test of time in the real world. For example, while carpet antimicrobials may inhibit mold in petri-dish tests, that is no guarantee that they will inhibit bacteria in real-world situations, as the OSHA ruling indicates.
One problem is that a variety of testing methods may be used to evaluate antimicrobial performance. According to Elliott Horner, Ph.D., microbiology lab director at Air Quality Sciences, which promotes its own newer test method for mold inhibition, “Many of those tests are decades old and some of them are rather simplistic. They don’t really give a good handle on how the product is going to perform,” he said, noting that some of the tests “were developed for the technical people in the company to use on a comparative basis, to test a new formulation” and may be fairly subjective. He added, “Unfortunately very few organizations have attempted to correlate the predictiveness of these tests with service conditions.”
The way those test results are often used in marketing contexts can also be misleading, argues Dobbin Callahan, manager of government markets for Tandus, a carpet manufacturer that has been critical of antimicrobial use. “Slathering agar solution [a common culture medium] on carpet and allowing it to grow under controlled heat and humidity proves only that agar solution grows mold and mildew under controlled heat and humidity,” he said, describing a common test procedure. “It’s not the carpet growing the mold, it’s the agar. It’s a non sequitur to conclude that carpet needs pesticides in order to perform well or for health reasons.”
The CDC report makes a broader point about marketing to the public: “The ”˜antibacterial’ label on household cleaning products, in particular, gives consumers the impression that the products perform ”˜better’ than comparable products without this labeling, when in fact all household cleaners have antibacterial properties.” In evaluating marketing claims of antimicrobial products, it is worth carefully evaluating what is being claimed, what is being implied, what is supported by evidence, and what testing or logic supports that evidence. Buyers may often find missing pieces at multiple levels.
Hygiene Without Pesticides
In sifting through claims to find products that meet their goals, those specifying or purchasing products can look to numerous products and designs without using antimicrobial pesticides. “My first pick is things that make life difficult for the colonizers,” building scientist Terry Brennan told EBN, “for example, fiberglass-faced gypsum board or fiber-cement board used on walls and floors that are going to receive tile, or in rooms where dampness or regular sweating is expected.”
Other products, in addition to not supporting mold or bacterial growth, offer some active protection against microbes without use of pesticides. Linoleum, for example, contains linseed oil, which continues to oxidize long after installation. On its website, Forbo claims that its linoleum offers bactericidal properties against a variety of microbes, including Salmonella and Staphylococcus. However, Marmoleum, Forbo’s linoleum, would seemingly need to be registered to make bactericidal claims, but Scott Day, marketing administrator for Forbo North America, said that pesticide regulations do not apply to Marmoleum..
Clay plaster is another natural product that appears to offer inherent mold-inhibition. Recent tests by American Clay, makers of American Clay Earth Plaster, showed that panels with applications of the plaster did not support mold or fungal growth, even when held for an extended period in a warm, humid environment. Croft Elsaesser, president of American Clay, attributed the effect to clay’s moisture-releasing properties: “Even though the clay holds moisture readily, given the opportunity to release that moisture it does that also,” he said. Remaining moisture destroys mold and its habitat. Elsaesser said that American Clay had been using borax as an antifungal additive to its plaster, but eliminated it following these tests, which showed equivalent performance with or without the borax.
Hydrated lime, or calcium hydroxide, is an age-old building material that has been used around the world in mortar, whitewash, plaster, and many other more specialized uses. Lime-based products like whitewash have traditionally been applied periodically to walls and other surfaces both inside and outside, where they temporarily increase the alkalinity of the surface, killing microbes. However, lime is caustic, and its antimicrobial effect is quickly spent, limiting its use. One interior paint, Caliwel, manufactured by the Alistagen Corporation, encapsulates lime, registered with EPA as a pesticide, in the paint to release it over time, prolonging its antimicrobial properties for four to six years, according to the company, which markets the paint for healthcare applications.
The Sittris BA chair is designed for cleanability, with its silicone upholstery. A silver antimicrobial treatment is an option on textile versions of the chair.
Other companies promote new products offering an inhospitable surface for microbial growth. The Sittris series of products, launched in 2007 for the healthcare market by furniture maker Keilhauer, uses silicone-upholstered seating (the largest silicone components that have ever been made, the company told EBN) to provide a very cleanable surface that does not support microbial growth and is inherently flame-retardant. (A textile surface containing silver antimicrobial is also an option.) The seating is designed for patient comfort and cleanability in healthcare settings. As opposed to antimicrobial surfaces that it didn’t find effective, the CDC report discussed earlier endorses the use of easily cleaned furniture, noting that it “reduces likelihood of disease.”
Are Antimicrobials Needed?
At the root of many of the concerns about the safety of antimicrobials, including health problems from exposure and antimicrobial resistance, is the sense that they’re not needed. Discussing antimicrobials in carpet, for example, Brennan said, “I don’t think it’s an advantage in carpet. You get the hazard from all phases of the pesticide and I don’t think you get a lot of benefit.” He added, “You might just get some benefit from [antimicrobials when] putting carpet on a basement floor,” a moisture-prone situation, but, he said, “you don’t get kisses for dumb boo-boos.” In other words, a superficial solution to an avoidable problem is misguided.
The danger of infection from building surfaces should also be put in context. As the Kaiser memo points out, quoting an industrial hygiene textbook, “the most common source of infectious agents is the patient’s own endogenous flora,” or microorganisms within one’s own body. The memo notes that these organisms usually don’t harm us, and are often beneficial, but when our immune systems are weakened we can become susceptible. Care should be taken to ensure that indoor environmental quality is not needlessly compromised by poor moisture control, for example, but, says the memo, “For environmental surfaces to be a source of infection, typically the patient’s immune system must be severely compromised.”
We can expect to see more antimicrobials, however. “There’s a market for it,” said Ron Swindle of Milliken. “There’s not always someone there to make sure that everything is spotless and perfectly maintained,” said Microban’s Swofford, adding, “They [antimicrobials] are a matter of convenience.”
Countering those claims, microbiologist Levy said, “To most of us, the potential hazards of use far outcry any potential benefit.” The potential for benefit is attractive: preventing rampant microbial growth in buildings is a worthy goal. But buyers should beware of products that claim too much, and, based on available evidence, many antimicrobial products may be oversold. If a manufacturer limits claims to protecting its own product, and there is a good reason to do so, it is likely on solid ground. A building product incorporating a pesticide and successfully making and defending a broader public health claim is harder to come by, however.
Checklist:
Improving Hygiene in Buildings
Asked if, as an industrial hygienist, she would listen to a pitch from a building product manufacturer making a public health claim backed up by studies, Kaiser’s Stewart said she would. “I think there is potential,” she said. “But we have to look at these claims very carefully to make sure that one solution doesn’t create another problem.”
For more information:
Centers for Disease Control and Prevention
“Guidelines for Environmental Infection Control in Health-Care Facilities”
www.cdc.gov/mmwr/preview/mmwrhtml/rr5210a1.htm
Kaiser Permanente
”Evaluation of Antimicrobial Property Claims in Finishes and Fabrics”
www.healthybuilding.net/healthcare/KP_Antimicrobial_Position_Paper.pdf
U.S. Environmental Protection Agency
Office of Pesticide Programs
www.epa.gov/pesticides/