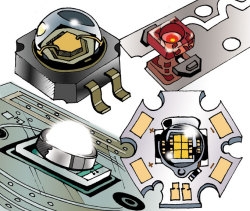
Light-emitting diodes (LEDs) use semi-conducting materials to turn electricity into light; electrons jump from one material to another, emitting photons as they travel. Different semiconductor materials create different colors of light: most white LEDs use indium gallium nitride (InGaN), which actually emits blue light. The blue light excites a phosphor coating on the lens of the diode, creating a yellow light that mixes with the blue and makes it look white to the human eye. Manufacturers also use closely placed red, green, and blue LEDs to deliver white light.
Light-emitting diodes (LEDs) use semi-conducting materials to turn electricity into light; electrons jump from one material to another, emitting photons as they travel. Different semiconductor materials create different colors of light: most white LEDs use indium gallium nitride (InGaN), which actually emits blue light. The blue light excites a phosphor coating on the lens of the diode, creating a yellow light that mixes with the blue and makes it look white to the human eye. Manufacturers also use closely placed red, green, and blue LEDs to deliver white light.
While LEDs use very little electricity, they also produce relatively small amounts of light. This light is highly concentrated and easy to focus, making it effective in some applications, such as task or display lighting. To use LEDs for area or ambient lighting, manufacturers collect multiple LEDs in a single fixture; getting enough output and an attractive color of light, however, has been challenging. Complicating matters, LEDs vary tremendously in quality; inexpensive LED lamps and flashlights tend to have inconsistent color temperature and light output.
When their light is effective, LEDs offer great potential for energy efficiency. Although current LED fixtures average 30”“40 lumens per watt (lpw), the Solid-State Lighting Program at the U.S. Department of Energy (DOE) estimates that LED fixtures are capable of achieving an efficacy of 160 lpw. By comparison, incandescent lamps typically produce 10”“18 lpw and compact fluorescent lamps (CFLs) 35”“60 lpw. LEDs also have the significant advantage of not containing mercury, which is a disposal and breakage hazard in fluorescent and mercury-vapor lamps.
LEDs have long lives; a fixture can last for a projected 35,000”“50,000 hours, compared to an incandescent lasting 750”“2,000 hours and a CFL lasting 8,000”“10,000 hours. In addition, LEDs rarely burn out totally (making them appealing in traffic signals and hard-to-reach locations), but their light output diminishes over time. DOE and industry representatives have defined the “useful life” of an LED as the time span over which it will produce at least 70% of its original light output.
Fixtures containing LEDs are becoming increasingly popular: colored plastic tubes containing LEDs have already widely replaced neon signs in new installations, and LEDs are now used in refrigerated display cases, where their directional light highlights products. LED fixtures for use in under-cabinet lighting and downlighting in living spaces are on their way to becoming cost-effective. As efficacies rise, costs decrease, and manufacturers get better at applying them to a variety of applications, LEDs should become an increasingly attractive option.
!ADVERTISEMENT!
For more information: