They are all around you! Most plastics, conductive polymers, and even medicines derive from molecules with a double bond between two carbon atoms, C=C. These molecules are called olefins and are mainly produced from fossil fuels through an energy-intensive and polluting process known as steam cracking. It requires temperatures of 800°C and produces the greenhouse gas carbon dioxide. Needless to day, alternatives to this process which could bring environmental and economic benefits are highly sought after.
They are all around you! Most plastics, conductive polymers, and even medicines derive from molecules with a double bond between two carbon atoms, C=C. These molecules are called olefins and are mainly produced from fossil fuels through an energy-intensive and polluting process known as steam cracking. It requires temperatures of 800°C and produces the greenhouse gas carbon dioxide. Needless to day, alternatives to this process which could bring environmental and economic benefits are highly sought after.
A team of researchers from the Center for Catalytic Hydrocarbon Functionalizations, within the Institute for Basic Science (IBS), in collaboration with Prof. Daniel J. Mindiola from the University of Pennsylvania, accomplished a reaction that was not possible before; they produced olefins with cheap readily available ingredients and at low temperature (75°C). This research outcome, published in Nature Chemistry, paves the way for an efficient use of natural gases to synthesize important chemical products.
Natural gases, such as methane and ethane, have strong carbon-hydrogen (C-H) bonds that are difficult to break. The research team managed to transform such unreactive molecules into olefins, the chemical feedstock of a myriad of products we use in our daily life.
This type of olefin production method is based on dehydrogenation, that is the removal of hydrogens which leads to the creation of the C=C bond, the mark of olefins. Since the energy required to break the strong C-H bonds is too high, the reaction can be accomplished only with the help of other molecules, called catalysts. Previously, dehydrogenation was possible only with catalysts based on expensive metals, like iridium.
Read more at Institute for Basic Science
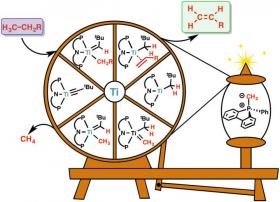
Image: Olefins, that is molecules with a double bond between carbons (C=C, green box) are generated from an unreactive molecules of natural gases (violet box). The reaction includes a carefully chosen titanium (Ti)-based catalyst, represented by the wheel and an additive molecule, pictured on the spool. The additive helps the wheel to spin, to recycle the catalyst back to its original form, so it can be used again to facilitate another reaction. Olefins are the chemical feedstock for a variety of other chemicals, like plastics, conductive polymers, medicines, etc. This reaction can be performed at low temperatures and it is the first time that it can be done cheaply. (Credit: IBS)