Lithium-air batteries are considered highly promising technologies for electric cars and portable electronic devices because of their potential for delivering a high energy output in proportion to their weight. But such batteries have some pretty serious drawbacks: They waste much of the injected energy as heat and degrade relatively quickly. They also require expensive extra components to pump oxygen gas in and out, in an open-cell configuration that is very different from conventional sealed batteries.
But a new variation of the battery chemistry, which could be used in a conventional, fully sealed battery, promises similar theoretical performance as lithium-air batteries, while overcoming all of these drawbacks.
The new battery concept, called a nanolithia cathode battery, is described in the journalNature Energy in a paper by Ju Li, the Battelle Energy Alliance Professor of Nuclear Science and Engineering at MIT; postdoc Zhi Zhu; and five others at MIT, Argonne National Laboratory, and Peking University in China.
Lithium-air batteries are considered highly promising technologies for electric cars and portable electronic devices because of their potential for delivering a high energy output in proportion to their weight. But such batteries have some pretty serious drawbacks: They waste much of the injected energy as heat and degrade relatively quickly. They also require expensive extra components to pump oxygen gas in and out, in an open-cell configuration that is very different from conventional sealed batteries.
But a new variation of the battery chemistry, which could be used in a conventional, fully sealed battery, promises similar theoretical performance as lithium-air batteries, while overcoming all of these drawbacks.
The new battery concept, called a nanolithia cathode battery, is described in the journalNature Energy in a paper by Ju Li, the Battelle Energy Alliance Professor of Nuclear Science and Engineering at MIT; postdoc Zhi Zhu; and five others at MIT, Argonne National Laboratory, and Peking University in China.
One of the shortcomings of lithium-air batteries, Li explains, is the mismatch between the voltages involved in charging and discharging the batteries. The batteries' output voltage is more than 1.2 volts lower than the voltage used to charge them, which represents a significant power loss incurred in each charging cycle. "You waste 30 percent of the electrical energy as heat in charging. ... It can actually burn if you charge it too fast," he says.
Continue reading at EurekAlert!
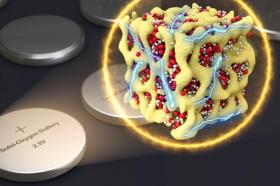
Image: Nanometer-scale particles made of lithium and oxygen compounds (depicted in red and white) are embedded in a sponge-like lattice (yellow) of cobalt oxide, which keeps them stable.
Credits: MIT Researchers