When the Deepwater Horizon oil rig suffered a catastrophic explosion and blowout on April 21, 2010, leading to the worst oil spill in the history of the petroleum industry, the well’s operators thought they would be able to block the leak within a few weeks. On May 9 they succeeded in lowering a 125-ton containment dome over the broken wellhead. If that measure had worked, it would have funneled the leaking oil into a pipe that carried it to a tanker ship above, thus preventing the ongoing leakage that made the spill so devastating. Why didn’t the containment work as expected?
The culprit was an icy mixture of frozen water and methane, called a methane clathrate. Because of the low temperatures and high pressure near the seafloor, the slushy mix built up inside the containment dome and blocked the outlet pipe, preventing it from redirecting the flow. If it hadn’t been for that methane clathrate, the containment might have worked, and four months of unabated leakage and widespread ecological devastation might have been prevented.
When the Deepwater Horizon oil rig suffered a catastrophic explosion and blowout on April 21, 2010, leading to the worst oil spill in the history of the petroleum industry, the well’s operators thought they would be able to block the leak within a few weeks. On May 9 they succeeded in lowering a 125-ton containment dome over the broken wellhead. If that measure had worked, it would have funneled the leaking oil into a pipe that carried it to a tanker ship above, thus preventing the ongoing leakage that made the spill so devastating. Why didn’t the containment work as expected?
The culprit was an icy mixture of frozen water and methane, called a methane clathrate. Because of the low temperatures and high pressure near the seafloor, the slushy mix built up inside the containment dome and blocked the outlet pipe, preventing it from redirecting the flow. If it hadn’t been for that methane clathrate, the containment might have worked, and four months of unabated leakage and widespread ecological devastation might have been prevented.
Now, a team of researchers at MIT has come up with a solution that might prevent such a disastrous outcome the next time such a leak occurs. It may also prevent blockages inside oil and gas pipelines that can lead to expensive shutdowns to clear a pipe, or worse, to pipeline rupture from a buildup of pressure.
Read more at Massachusetts Institute of Technology
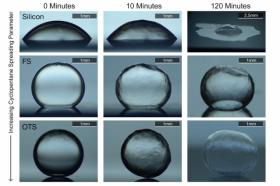
Image: A new surface coating developed by Kripa Varanasi and his team causes water to bead up on the inner surface of a pipe rather than spreading out. This prevents the formation of ices that could lead to a clog in an oil pipeline or well. (Credits: Image courtesy of the researchers)