
Consumers whose drinking water can be contaminated by the release of untreated wastewater after heavy rains face increased risk for gastrointestinal illness, according to a report in the journal Environmental Health Perspectives. “Combined” sewer systems collect both sewage and stormwater runoff on the way to treatment facilities. When heavy rainfall fills these systems beyond their capacity, untreated wastewater can back up into homes. To reduce the risk of home flooding during heavy precipitation, municipalities often discharge some of the untreated flow into nearby bodies of water. The release of untreated waste is known as a combined sewer overflow.
>> Read the Full Article

Do you have any idea just how many organizims are in seawater? Not the fish you can see, but the microscopic organizims you cant see?
Dip a beaker into any portion of the world’s oceans, and you’re likely to pull up a swirling mix of planktonic inhabitants. The oceans are teeming with more than 5,000 species of phytoplankton — microscopic plants in a kaleidoscope of shapes and sizes. Together, phytoplankton anchor the ocean’s food chain, supplying nutrients to everything from single-celled organisms on up to fish and whales.
Through photosynthesis, these tiny organisms supply more than half the world’s oxygen. When these plants die, they drift to the ocean bottom, or evaporate into the air as carbon — a process that generates more than half the world’s cycling carbon.
>> Read the Full Article

Emergency hospital admissions rise following heavy rainfall after a new study found consumers’ drinking water can be contaminated by the release of untreated wastewater, increasing the risk for gastrointestinal illness.
"Combined" sewer systems collect both sewage and stormwater runoff on the way to treatment facilities. When heavy rainfall fills these systems beyond their capacity, untreated wastewater can back up into homes.
To reduce the risk of home flooding during heavy precipitation, municipalities often discharge some of the untreated flow into nearby bodies of water.
>> Read the Full Article

Strange as it sounds, flood control can be part of the solution to managing California’s droughts. University of California scientists have shown that making more room for floodwaters can improve the state’s groundwater supplies and fisheries.
Removing some levees or rebuilding aging ones some distance away from riverbanks can appreciably replenish aquifers during wet years, providing some relief during droughts.
>> Read the Full Article
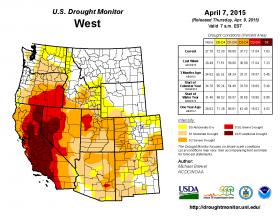
Nine states report record low snowpacks. A report from the US Department of Agriculture states, “the largest snowpack deficits are in record territory for many basins,especially in the Cascades and Sierra Nevada where single – digit percent of normal conditions prevail. Very low snowpacks are reported in most of Washington, all of Oregon, Nevada, California, parts of Arizona, much of Idaho, parts of New Mexico, three basins in Wyoming, one basin in Montana, and most of Utah.” This region is undergoing the warmest winter temperatures since record keeping began in 1895.
>> Read the Full Article

The number of gorillas and chimpanzees in Central Africa continues to decline due to poaching, habitat loss and disease according to a new plan published by WWF, International Union for Conservation of Nature, Wildlife Conservation Society and partners. The strategy, “Regional Action Plan for the Conservation of Western Lowland Gorillas and Central Chimpanzees 2015-2025”—outlines the growing number of threats to these great apes across six range countries, including gaps in law enforcement and the threats by well-connected traffickers seeking to supply the illegal commercial market.
>> Read the Full Article

Alarm bells are ringing for Arctic wildlife with the discovery that mercury levels in the feathers of ivory gulls have increased almost 50-fold.
University of Saskatchewan biologists studied the feathers of museum specimens spanning a 130-year period. Lead researcher Dr Alex Bond told BBC News, “We’re concerned because the mercury’s going up but their diet hasn’t changed over the 130 years we’ve studied. It’s gone up 45 times, which is twice the average for an animal species in the Arctic.”
>> Read the Full Article

Supercomputers and genetic engineering could help boost crops’ ability to convert sunlight into energy and tackle looming food shortages, according to a team of researchers. Photosynthesis is far from its theoretical maximum efficiency, say the authors of a paper in Cell, published on 26 March. They say that supercomputing advances could allow scientists to model every stage in the process and identify bottlenecks in improving plant growth.
>> Read the Full Article

 ENN
Environmental News Network -- Know Your Environment
ENN
Environmental News Network -- Know Your Environment