Changing the natural electrical signaling that exists in cells outside the nervous system can improve resistance to life-threatening bacterial infections, according to new research from Tufts University biologists. The researchers found that administering drugs, including those already used in humans for other purposes, to make the cell interior more negatively charged strengthens tadpoles’ innate immune response to E. coli infection and injury. This reveals a novel aspect of the immune system – regulation by non-neural bioelectricity – and suggests a new approach for clinical applications in human medicine. The study is published online May 26, 2017, in npj Regenerative Medicine, a Nature Research journal.
“All cells, not just nerve cells, naturally generate and receive electrical signals. Being able to regulate such non-neural bioelectricity with the many ion channel and neurotransmitter drugs that are already human-approved gives us an amazing new toolkit to augment the immune system’s ability to resist infections,” said the paper’s corresponding author Michael Levin, Ph.D., Vannevar Bush professor of biology and director of the Allen Discovery Center at Tufts and the Tufts Center for Regenerative and Developmental Biology in the School of Arts and Sciences. Levin is also an associate faculty member of the Wyss Institute of Biologically Inspired Engineering at Harvard University.
Changing the natural electrical signaling that exists in cells outside the nervous system can improve resistance to life-threatening bacterial infections, according to new research from Tufts University biologists. The researchers found that administering drugs, including those already used in humans for other purposes, to make the cell interior more negatively charged strengthens tadpoles’ innate immune response to E. coli infection and injury. This reveals a novel aspect of the immune system – regulation by non-neural bioelectricity – and suggests a new approach for clinical applications in human medicine. The study is published online May 26, 2017, in npj Regenerative Medicine, a Nature Research journal.
“All cells, not just nerve cells, naturally generate and receive electrical signals. Being able to regulate such non-neural bioelectricity with the many ion channel and neurotransmitter drugs that are already human-approved gives us an amazing new toolkit to augment the immune system’s ability to resist infections,” said the paper’s corresponding author Michael Levin, Ph.D., Vannevar Bush professor of biology and director of the Allen Discovery Center at Tufts and the Tufts Center for Regenerative and Developmental Biology in the School of Arts and Sciences. Levin is also an associate faculty member of the Wyss Institute of Biologically Inspired Engineering at Harvard University.
All vertebrates, from fish to people, have two kinds of immunity with common features. The adaptive immune system relies on the memory of previous exposure to a specific pathogen and is the basis for current vaccination strategies. The innate immune system is present from the time an egg is fertilized and provides a first line of defense against pathogens through surface barriers, antimicrobial amino acids called peptides, and certain blood cells. The innate immune system also plays a role in tissue repair and regeneration, and the interplay between regeneration and innate immunity is an emerging field of study.
Continue reading at Tufts University
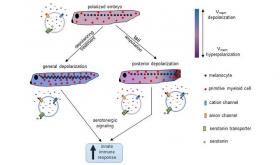
Image: How Vmem signal strengthens innate immune response: Normal tadpoles have polarized cells, with specific native amounts and distributions of melanocytes (pigment cells) and primitive myeloid cells (part of the innate immune system). Following chemical or genetic treatments that depolarize the cells' Vmem (transmembrane potential, or voltage potential caused by differences in negative and positive ions on opposite sides of a cell's membrane), pathways involving serotonin signaling induce proliferation and redistribution of both melanocytes and primitive myeloid cells, leading to an increase in the efficiency of the immune response when stimulated with a pathogen such as E. coli. Tail amputation induces a strong posterior Vmem depolarization at the site of injury, where melanocytes and primitive myeloid cells are recruited, resulting in a net increase of the latter in the embryo, leading to an enhanced innate immune response. Findings from Tufts University biologists appear in npj Regenerative Medicine on May 26, 2017. (Credit: Jean-Francois Pare / Tufts University)