Methane is present in the natural gas that is very abundant in the earth's crust and has found many uses in modern applications, mainly as a burning fuel.
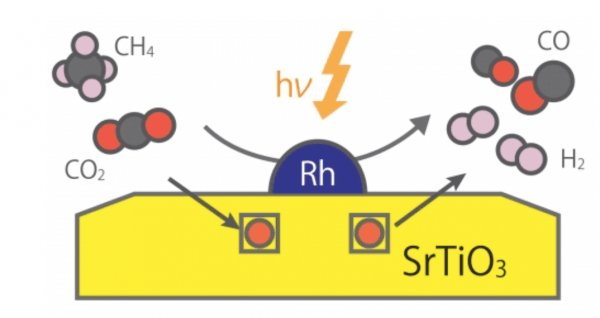
Methane is present in the natural gas that is very abundant in the earth's crust and has found many uses in modern applications, mainly as a burning fuel. Alternatively, methane can be converted into a useful mixture of hydrogen and carbon monoxide, called "synthesis gas," by reaction with carbon dioxide in what is referred to as dry reforming of methane (DRM).
This DRM reaction is termed "uphill" because it requires the consumption of external energy: thermal reactors have to be at a high temperature of more than 800 °C for efficient conversion. Reaching such high temperatures requires burning other fuels, resulting in massive greenhouse gas emissions, which are the leading cause of climate change. In addition, the use of high temperatures also causes the deactivation of commonly used catalysts due to aggregation and carbon precipitation—so-called coking.
Instead of dealing with such drawbacks of thermal catalysis systems for DRM reaction, researchers have attempted to drive the conversion of methane at dramatically lower temperatures using photocatalysts activated by light. Although various photocatalyst-like materials have been proposed, it has proven challenging to obtain acceptable conversion performance at low temperatures.
Read more at Kyushu University
Image: Photocatalytic uphill conversion of natural gas beyond thermal reaction systems. CREDIT: Kyushu University