A novel molybdenum-coated catalyst that can efficiently split water in acidic electrolytes is developed by researchers at KAUST and could help with efficient production of hydrogen.
A novel molybdenum-coated catalyst that can efficiently split water in acidic electrolytes is developed by researchers at KAUST and could help with efficient production of hydrogen.
When burned, hydrogen is converted into water and heat to make an entirely clean power source. Thus, in the quest for greener power, there is an urgent need for a sustainable and efficient means of producing it. One way is to split water using a process known as photocatalytic hydrogen evolution: water molecules are split into hydrogen and oxygen using only sunlight to provide the necessary energy. In this sense, hydrogen acts as a means of storing solar energy.
Scientists are searching for ways of improving this water-splitting reaction by developing an optimal catalyst. While many different materials have been tried, they are usually adversely affected by the oxygen that is also created alongside the hydrogen during the process. The two gaseous products can easily recombine back to water due to reverse water-forming reactions, hindering the production of hydrogen.
Dr. Angel Garcia-Esparza and Dr. Tatsuya Shinagawa—two former KAUST Ph.D. students as leading researchers supervised by Associate Professor of Chemical Science Kazuhiro Takanabe—collaborated with colleagues from the Catalysis Center and other specialists in the University to create a hydrogen-evolution reaction catalyst that is both acid-tolerant and selectively prevents the water-reforming reaction1.
Read more at King Abdullah University of Science & Technology (KAUST)
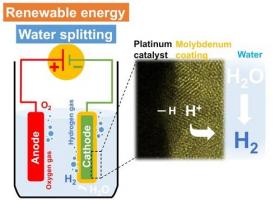
Image: A high-resolution electron microscope image (right) of the platinum electrocatalytic layer coated with molybdenum. The platinum catalyzes the hydrogen-evolution reaction (left) in acidic medium from protons in the electrolyte while the molybdenum layer inhibits water-forming reactions. (Credit: © 2017 KAUST)