Global climate change and the energy crisis mean that alternatives to fossil fuels are urgently needed. Among the cleanest low-carbon fuels is hydrogen, which can react with oxygen to release energy, emitting nothing more harmful than water (H2O) as the product. However, most hydrogen on earth is already locked into H2O (or other molecules), and cannot be used for power.
Global climate change and the energy crisis mean that alternatives to fossil fuels are urgently needed. Among the cleanest low-carbon fuels is hydrogen, which can react with oxygen to release energy, emitting nothing more harmful than water (H2O) as the product. However, most hydrogen on earth is already locked into H2O (or other molecules), and cannot be used for power.
Hydrogen can be generated by splitting H2O, but this uses more energy than the produced hydrogen can give back. Water splitting is often driven by solar power, so-called “solar-to-hydrogen” conversion. Materials like titanium oxide, known as semiconductors with the wide band-gap, are traditionally used to convert sunlight to chemical energy for the photocatalytic reaction. However, these materials are inefficient because only the ultraviolet (UV) part of light is absorbed—the rest spectrum of sunlight is wasted.
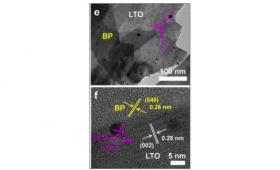
Now, a team in Osaka University has developed a material to harvest a broader spectrum of sunlight. The three-part composites of this material maximize both absorbing light and its efficiency for water splitting. The core is a traditional semiconductor, lanthanum titanium oxide (LTO). The LTO surface is partly coated with tiny specks of gold, known as nanoparticles. Finally, the gold-covered LTO is mixed with ultrathin sheets of the element black phosphorus (BP), which acts as a light absorber.
“BP is a wonderful material for solar applications, because we can tune the frequency of light just by varying its thickness, from ultrathin to bulk,” the team leader Tetsuro Majima says. “This allows our new material to absorb visible and even near infrared light, which we could never achieve with LTO alone.”
Read more at Osaka University
Image: Electron microscope images of visible-NIR light responsible photocatalyst composed with black phosphorous (BP), lanthanum titanate (LA2Ti2O7, LTO), and gold nanoparticles (Au). (Credit: Zhu M, Cai X, Fujitsuka M, Zhang J, Majima T, Angewandte Chemie: International Edition 56 (2017))