Magnesium batteries offer promise for safely powering modern life – unlike traditional lithium ion batteries, they are not flammable or subject to exploding – but their ability to store energy has been limited.
Researchers reported Aug. 24 in the journal Nature Communications the discovery of a new design for the battery cathode, drastically increasing the storage capacity and upending conventional wisdom that the magnesium-chloride bond must be broken before inserting magnesium into the host.
Magnesium batteries offer promise for safely powering modern life – unlike traditional lithium ion batteries, they are not flammable or subject to exploding – but their ability to store energy has been limited.
Researchers reported Aug. 24 in the journal Nature Communications the discovery of a new design for the battery cathode, drastically increasing the storage capacity and upending conventional wisdom that the magnesium-chloride bond must be broken before inserting magnesium into the host.
“We are combining a nanostructured cathode and a new understanding of the magnesium electrolyte,” said Yan Yao, associate professor of electrical and computer engineering at the University of Houston and lead author on the paper. “That’s new.”
The work was first conceived by Yao and postdoctoral fellow Hyun Deog Yoo in 2014; the project spanned several years and involved scientists from three universities and three national laboratories, working both experimentally and theoretically.
Read more at University of Houston
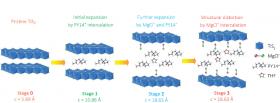
Image: This schematic shows the structural evolution of titanium disulfide at different stages of intercalation. Interlayers are expanded or distorted as different amounts of pillaring molecules, complex cations and solvents are intercalated into the van der Waals gap of a host material at each stage. Credit: University of Houston, Department of Electrical and Computer Engineering