The world’s first widespread human testing of a flu vaccine which researchers hope will protect more over 65-year-olds against influenza has begun in the NHS.
The world’s first widespread human testing of a flu vaccine which researchers hope will protect more over 65-year-olds against influenza has begun in the NHS.
More than 10,000 people aged 65 and over will be asked to take part in a study supported by the National Institute for Health Research (NIHR) and delivered by the University of Oxford in Berkshire and Oxfordshire. The recruitment target is 500. Researchers believe the vaccine could have a major impact on the worldwide fight against the virus, which affects about a billion people worldwide a year with 250,000 to 500,000 annual deaths, mainly in the over-65 age group. Current vaccines are only effective in 30 to 40% of over 65s as the immune system weakens with age and researchers believe the new vaccine could increase this. For those who receive the jab but still get the flu, researchers believe the new vaccine could also reduce the severity and duration of the illness.
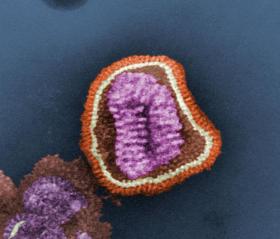
It is believed the vaccine will offer a stronger protection against flu because it uses a different mechanism to get the body to protect against the virus. Under the microscope, the flu virus looks like a spherical cushion with lots of pins sticking out of it. The existing flu vaccines use surface proteins that lie on the outside of flu cells – the heads of the pins - to stimulate the body’s immune system to produce disease-fighting antibodies. But as the virus changes each year, so do the surface proteins, haemagglutinin and neuraminidase, meaning the flu vaccine needs to change too.
Read more at University of Oxford
Photo credit: Cynthia Goldsmith (CDC) via Wikimedia Commons