One of the strongest chemical bonds in organic chemistry is formed between carbon and fluorine, giving unique properties to chemical compounds featuring this group. Pharmaceutical researchers are very interested in carbon-fluorine bond containing molecules because of the way they mimic certain behaviors of biological compounds. However, the strength of the carbon-fluorine bond makes it difficult to remove and replace fluorine atoms in a molecule, greatly limiting the structures and types of chemicals that can be made.
One of the strongest chemical bonds in organic chemistry is formed between carbon and fluorine, giving unique properties to chemical compounds featuring this group. Pharmaceutical researchers are very interested in carbon-fluorine bond containing molecules because of the way they mimic certain behaviors of biological compounds. However, the strength of the carbon-fluorine bond makes it difficult to remove and replace fluorine atoms in a molecule, greatly limiting the structures and types of chemicals that can be made.
Now, a team of Japanese scientists from Osaka University, in collaboration with the RIKEN Center for Life Science Technologies and Tokyo Medical and Dental University, has developed a new way of extending the chemistry of fluoroalkene molecules, granting access to a new range of fluoroalkenes for pharmaceuticals and other applications. They recently reported their findings in the Journal of the American Chemical Society.
“It’s easy to add fluorine groups to alkenes, but not so easy to change them later,” first author Hironobu Sakaguchi says. “We previously showed that tetrafluoroethylene (TFE), one of the most important materials for the production of fluorine-containing polymers such as Teflon™ in the fluorine industry, could be transformed into trifluorovinyl compounds using transition-metal complexes, although the scope of those reactions was quite limited. Furthermore, it was hard for those reactions to be applicable to other fluoroalkenes than TFE. Now, we can use copper-catalyzed conditions which are applicable to versatile fluoroalkenes to replace fluorine atoms with boron, which opens up a huge range of potential target molecules and should boost the use of fluoroalkenes in pharmacological studies.”
Read more at Osaka University
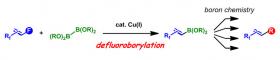
Image: Synthetic route to diverse fluoroalkenes via defluoroborylation of polyfluoroalkenes.
(©2017 Osaka University, RIKEN, and Tokyo Medical and Dental University)