The direct oxidation of methane—found in natural gas—into methanol at low temperatures has long been a holy grail. Now, researchers at Tufts have found a breakthrough way to accomplish the feat using a heterogeneous catalyst and cheap molecular oxygen, according to a paper published today in the journal Nature by a team led by Tufts University chemical engineers.
The direct oxidation of methane—found in natural gas—into methanol at low temperatures has long been a holy grail. Now, researchers at Tufts have found a breakthrough way to accomplish the feat using a heterogeneous catalyst and cheap molecular oxygen, according to a paper published today in the journal Nature by a team led by Tufts University chemical engineers.
Methanol is a key feedstock for the production of chemicals, some of which are used to make products such as plastics, plywood and paints. Methanol also can fuel vehicles or be reformed to produce high-grade hydrogen for fuel cells.
However, the current method for producing methanol from methane- or coal-derived synthesis gas involves a multi-step process that is neither efficient nor economical in small-scale applications. As a result, methane emissions from oil wells, accounting for 210 billion cubic feet of natural gas annually, are being vented and flared, according to the U.S. Energy Information Administration. Meanwhile, the growth of hydraulic fracturing, or fracking, and the subsequent use of shale gas, the chief component of which is methane, have dramatically increased the natural gas supply in the United States, and accelerated the desire to upgrade methane into more valuable chemicals, such as through oxidation to methanol or carbonylation to acetic acid.
As a result, scientists have been seeking more efficient and less expensive ways to convert methane with a process that uses inexpensive molecular oxygen in mild conditions in which relatively low temperatures and pressures are used. The potential benefit is significant. In 2000, the availability of cheap shale gas represented just 1 percent of American natural gas supplies, while today it represents more than 60 percent.
Read more at Tufts University
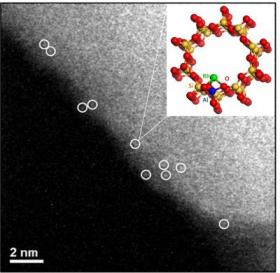
Image: Aberration-corrected HAADF/STEM images of as-synthesized Rh-ZSM-5. Single rhodium cations are circled in white with proposed ball-stick model of the structure. (Credit: Lawrence F. Allard, co-author and researcher at Oak Ridge National Laboratory)